Some microbes can be quite clingy.
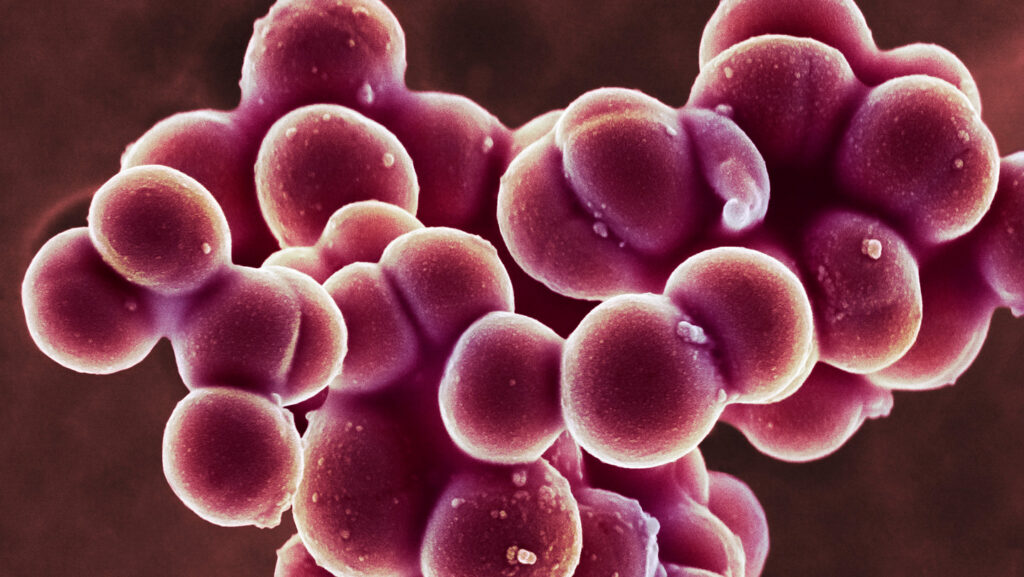
Staphylococcus aureus, a bacterial species responsible for staph infections, latches onto human skin with one of the strongest biological bonds ever recorded, researchers report in the Sept. 5 Science Advances. This powerful grasp is strengthened by the mineral calcium, preventing bacterial cells from being washed or brushed away from skin.
An influx of calcium to damaged skin, such as a cut or a condition like eczema, is “a way of growing your skin faster,” says Rafael Bernardi, a biophysicist at Auburn University in Alabama. But “the mechanism that we use to fix a bruise is the same mechanism that the bacteria take advantage of to bind better to our skin.”
A common skin microbe, S. aureus can invade the body through cuts and scrapes to cause skin infections such as cellulitis or life-threatening systemic infections when staph spreads to other parts of the body. Disrupting the super strong bond between staph and skin could help researchers develop new treatments.
Bernardi and colleagues used microscopy and computer simulations to analyze the molecular handshake between a bacterial protein called SdrD and a human protein called DSG-1 in lab dishes. In the presence of calcium, the two proteins could withstand forces stronger than 2 nanonewtons, or 2 billionths of a newton. “We don’t know of any [biological] bond that is stronger than this one without a covalent bond,” which is when two atoms share electrons, Bernardi says.
Mechanical forces going on inside a cell are typically weaker, measured in no more than dozens of piconewtons (a thousandth of a nanonewton), Bernardi says. It might take 60 to 80 piconewtons, for instance, to break apart proteins that help muscles contract. But SdrD and DSG-1 cling together roughly 20 times more strongly. At the molecular level, tiny bacteria “can grip more than your muscles can lift,” Bernardi says.
People often have different strains of staph living on their skin, and it’s unclear if there are variations in binding strength among strains. Still, preventing SdrD and DSG-1 from binding could make staph infections easier to treat.
Some antibiotic-resistant strains such as MRSA can form biofilms, a protective, slimy shield that forms around clusters of bacteria and can make infections harder to treat. But if individual cells never attach to skin, Bernardi says, then the biofilm can’t develop, and immune cells, or antibiotics, can swoop in for the kill.
Read the full article here