A strange type of ice thought to dwell deep in the oceans of alien planets has finally been proven to exist.
For the first time, researchers have directly observed a sort of hybrid phase of water called plastic ice, which forms at high temperatures and pressures and exhibits traits of both solid ice and liquid water. The observations, reported February 12 in Nature, may help researchers better understand the internal architecture and processes of other worlds in our solar system and beyond, some of which might be habitable.
Plastic ice is “something intermediate between a liquid and a crystal, you can imagine that it is softer when you squeeze it,” says physicist Livia Bove of Sapienza University of Rome. It’s called plastic ice because it is more easily molded or deformed than typical crystalline ice, exhibiting a property scientists call plasticity, she says. “Like something that can [squeeze] through a hole and come out, even if it’s still solid.”
Most of the ice on Earth’s surface — including ice cubes, glaciers and snow — consists of water molecules arranged in a hexagonal lattice that resembles a honeycomb. Scientists classify this common ice as ice lh. But in addition to ice Ih, there are at least 20 other known ice phases that form in different pressure and temperature conditions. At pressures above 20,000 bars — or 20,000 kilograms per square inch — ice lattices compress into Ice VII, a polymorph with a dense, cubic structure in which molecules are ordered like the cubies in a Rubik’s Cube. Ice VII has been found trapped in diamonds originating from Earth’s mantle and is thought to occur inside other planets too. And Kurt Vonnegut fans may be interested to hear that an ice IX was discovered in 1996, though it lacks the terrifying ability to freeze entire oceans.
There are also ice phases that have only been theorized to exist. Over 15 years ago, computer simulations showed that when ice VII is heated and subjected to extreme pressures, its individual water molecules should start to rotate freely, as if a liquid, while occupying fixed positions, as in a solid. Since the hypothetical phase shared the same cubic crystal structure as ice VII, it became known as plastic ice VII. But because performing experiments at such high pressures was technically infeasible at the time, solid evidence of plastic ice’s existence eluded scientists for years.
For the new study, Bove and colleagues utilized a relatively new tool at the Institut Laue-Langevin in Grenoble, France that’s able to measure the motions of molecules under extreme pressures. In experiments, they pointed a neutron beam at water samples and subjected the samples to temperatures up to 326° C and pressures up to 60,000 bars. As the incoming neutrons interacted with the water molecules in the samples, they gained or lost energy depending on how much the water molecules were moving and rotating, before being scattered away toward a detector. Measuring the scattered neutrons’ energies allowed Bove’s team to characterize the molecules’ motions and identify the phase that had formed.
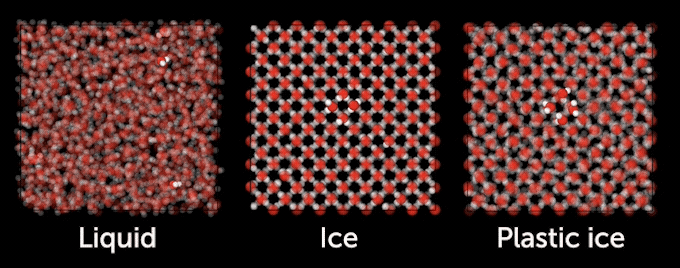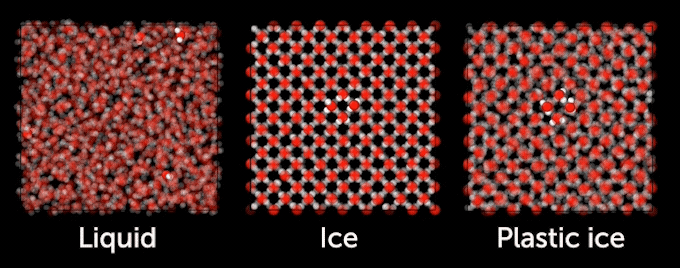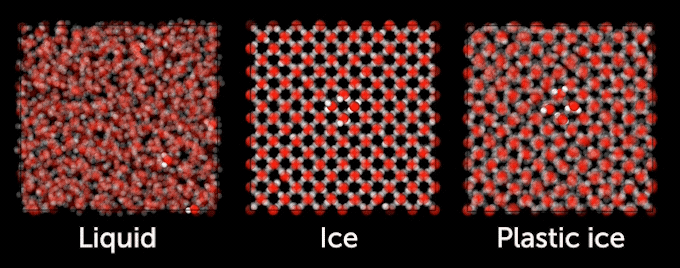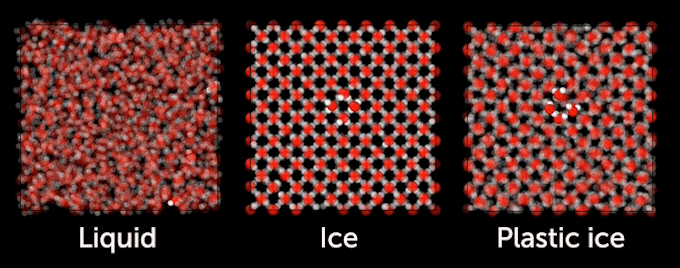
Above 177° C and over roughly 30,000 bars — about 28 times the pressure at the deepest point in Earth’s oceans — Bove’s team observed a phase of ice that possessed a cubic crystal lattice with water molecules rotating about as fast as those in liquid water. They identified the phase as plastic ice VII, finally confirming its existence.
However, one observed detail diverged from predictions. Rather than revolve freely, the water molecules appeared to swivel in jerky motions. As the molecules rotate, they break their hydrogen bonds with one neighbor only to rapidly turn and bond with another, Bove explains.
Plastic ice VII may have existed during the early formational stages of Europa, Titan and other icy moons in our solar system, before all the water had escaped from their high-pressure interiors, says planetary scientist Baptiste Journaux of the University of Washington in Seattle. The new observations could help researchers piece together the story of how these moons evolved into the ocean worlds they are today, he says.
And beyond our solar system, the strange ice may repose at the bottom of giant oceans on exoplanets, some of which are thousands of kilometers deep and potentially habitable, Journaux says. Investigating how easily plastic ice VII incorporates salts into its lattice could help determine whether the strange phase’s presence enhances the exchange of salts between exoplanet seafloors and the oceans above, he says. “That would actually feed the ocean with more nutrients.”
Read the full article here