Published on
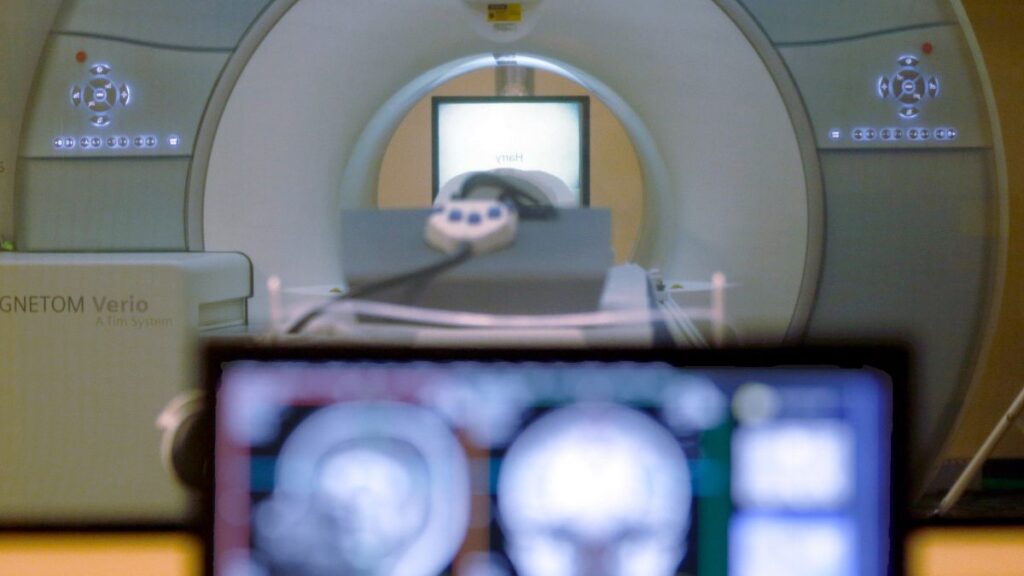
The European Commission has formally introduced restrictions previously reported by Euronews in response to what it describes as discriminatory barriers imposed by China against European medical device manufacturers.
Following a detailed investigation, the Commission found “clear evidence” that China had been unfairly blocking EU-made medical devices from its public procurement market.
This marks the first countermeasure taken under the International Procurement Instrument (IPI), which came into force in August 2022 to promote fair access for EU firms to procurement opportunities outside the bloc.
“Our aim with these measures is to level the playing field for EU businesses. We remain committed to dialogue with China to resolve these issues,” said Trade Commissioner Maroš Šefčovič.
Under the new rules, Chinese companies are barred from bidding on public contracts for medical devices in the EU single market that exceed €5 million. Additionally, successful bids must contain no more than 50% of inputs originating from China.
According to the Commission, the measures are proportionate to China’s own restrictions and are designed to ensure the continued availability of critical medical equipment for EU healthcare systems. Exceptions will apply in cases where no viable alternative suppliers are available.
The Commission pointed out that the decision aligns with international trade obligations, including those under the World Trade Organization (WTO), noting that the EU has no binding procurement commitments with China.
EU-based medical device companies have long struggled to access China’s procurement market, despite China being one of the bloc’s largest export destinations for such products, accounting for 11% of exports in 2022.
The Commission’s investigation focused on China’s government procurement law, which enforces a “Buy China” policy, requiring public institutions to prioritise domestic products and services.
The probe identified several barriers faced by EU firms, including opaque approval processes, discriminatory certification practices, ambiguous national interest clauses used to exclude foreign suppliers, and unsustainable pricing requirements.
According to a 2025 Commission report, 87% of public procurement contracts for medical devices in China were subject to exclusionary and discriminatory practices against EU suppliers.
The new EU measures come at a delicate moment in EU-China relations, which are undergoing a cautious diplomatic reset.
Both sides have intensified efforts to manage longstanding disputes amid shifting global dynamics, including the aftermath of the Trump-era trade wars and ongoing US-China tensions.
A key milestone in this renewed dialogue is the upcoming EU-China Summit, now scheduled to take place in Beijing in the second half of July 2025.
Meanwhile, reciprocal actions continue to define the trade relationship. China has extended its anti-dumping investigation into EU pork imports by six months, while the EU recently imposed tariffs of up to 45% on Chinese electric vehicles (EVs), reflecting a strategic pattern of targeting politically sensitive industries ahead of high-level negotiations.
Read the full article here